About Macsa
Coding, marking and traceability solutions
Macsa ID provides our clients with solutions concerning their coding, identification and traceability needs. We combine different technologies such as hardware and software, services, consumables and spare parts for companies in a wide range of sectors such as the food and beverage, cosmetics, pharmaceutical, automotive or electronics industries.
Macsa ID is formed by 4 business units:

Hardware
Our own production of equipment dedicated to coding and traceability, industrial marking and product labelling. Various technologies such as lasers, labellers and high resolution machines, thus covering all the coding requirements of the packaging and industrial markets.

Software
Our own production of equipment dedicated to coding and traceability, industrial marking and product labelling. Various technologies such as lasers, labellers and high resolution machines, thus covering all the coding requirements of the packaging and industrial markets.

Services
Our own production of equipment dedicated to coding and traceability, industrial marking and product labelling. Various technologies such as lasers, labellers and high resolution machines, thus covering all the coding requirements of the packaging and industrial markets.

Consumables
Our own production of equipment dedicated to coding and traceability, industrial marking and product labelling. Various technologies such as lasers, labellers and high resolution machines, thus covering all the coding requirements of the packaging and industrial markets.
Traceability guarantee
Macsa ID is a private company with a clear mission:
Helping the manufacturing industry to secure their products and their customers’ peace of mind while ensuring a brighter tomorrow for our future generations.
All this thanks to continuous investment in technological innovation (R&D) as well as investing in value-added sales channels. These technologies specifically address the coding, identification and traceability needs of our clients, enabling them to protect their brands by:
· Ensuring the integrity of their supply chains,
· Complying with regulations related to the safe use of their products.
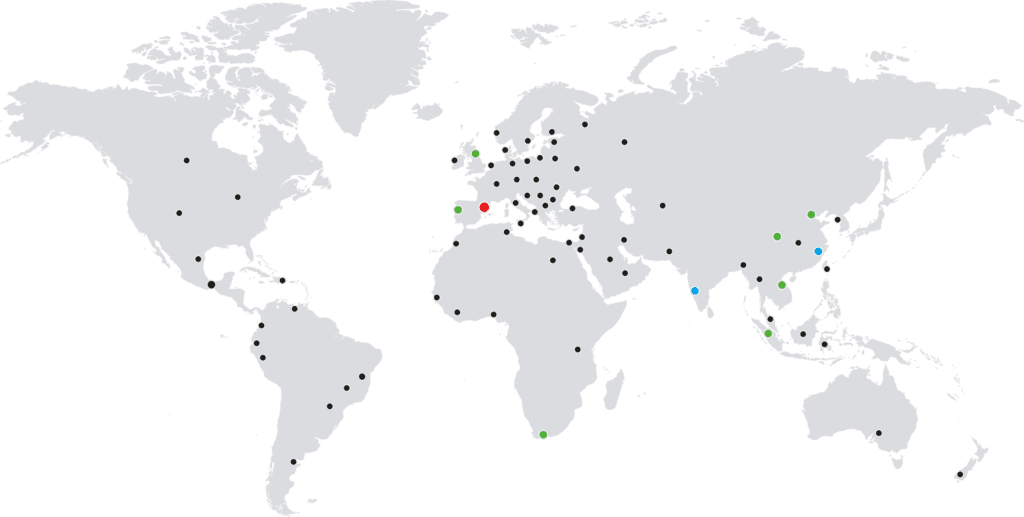
Presence in more than 90 countries
We are a company with a clear international vocation based in Barcelona, with 12 technical-commercial centers strategically located throughout Spain and a presence in over 90 countries.
Currently, we have 3 production plants in Spain, China, and India, as well as our own subsidiaries in Portugal, the United Kingdom, Malaysia, China, India, and South Africa.
In a global and highly competitive environment, Macsa ID becomes a trusted partner, capable of providing worldwide coverage and a strong range of services across the globe.

United Kingdon
Thanks to the acquisition of Halo Lasers in the United Kingdom, an innovative manufacturer of lasers for industrial marking, Macsa ID consolidates its technological leadership in the industrial sector at a global level and open its own office in this important country. The Macsa UK team is made up of 9 people.

China
At Macsa ID Coding Technology in Schenzhen (China) 25 people are working: 10 commercials, 12 technicians, 2 staff and a general manager. Macsa ID has provided sales and service from this once since 2013. In 2018 Macsa opened a manufacturing facility in order to serve the ASPAC markets with competitively priced products with improved delivery times. These 2 fundamental competitive advantages have further strengthened Macsa’s market position

Malaysia
Macsa Malaysian Kuala Lumpur Office includes a Business Director and a technician, they provide commercial and technical service to the Asia- Pacific area (except China). Our facilities are designed to provide full service to our customers, from laboratory samples, service repairs and spare parts stock. Also training sessions are carried out. Malaysia was chosen as a venue in Asia for its central location, good level of English and great connections to other countries.

Portugal
Macsa ID has presence in Portugal since 12 years ago through its direct subsidiary. There’s a commercial, technical and administrative workforce capable to provide careful attention to the needs of their customers. Macsa ID is active in all sectors including food, beverage, automotive, pharmaceutical or Logistics, and work with some of the main companies in the country such as Rar, Sogrape, Navigator Company, Tyco, Delphi, Ikea, Quiterios, Exporlux, “Guerreiro costa, Politejo, Colep, …”
100 years of history
Macsa ID has its origins in the company Framun, a manufacturer and distributor of rubber stamps that was founded in Manresa in 1908. In 1983, taking advantage of the latest advances in coding and marking technologies, it was established as an independent line of the company, dedicating itself to the sale, supply and service of coding products for Spain. In 1989, Macsa ID started to investigate the potential of laser coding and marking technology in Spain, and subsequently, in 1995, its role as industry leader in terms of marking and integration of coding systems had already been consolidated. From 1996 onwards, the equipment developed by Macsa ID began to be distributed in Germany and after only a decade was present in 18 countries worldwide. Since then, the continued progress of the company has allowed annual and biennial launches.
In 2007, the new F-9000 Fiber laser was launched and, after a couple of years, a double launch: iCON, the first small-sized laser encoder and the fastest coding laser in the world, the KIP 107.
In 2012 the coding, marking and identification process was centralised with the Integra Optima software, in addition to launching the Nano laser, a compact and affordable industrial laser and the K-1000 HPD for pre-cutting and micro-perforation.
In 2013, the market’s most versatile labeller was launched, idBlocks, the modular label printer available to mark boxes, sacks or pallets. After a couple of years, two very important lasers currently in the range of lasers offered by Macsa ID, saw the light: the new iCON2, the renewed version of the legendary Macsa ID iCON.
In 2015, we consolidated the new division of Macsa Textile, offering sustainable laser solutions for the denim market. In 2016, we introduced the new range of idJET, high-resolution marking solutions ideal for industrial and packaging applications. The opening of our new subsidiary in the United Kingdom in 2017 was celebrated with the launch of a new range of industrial lasers and workstations, a perfect combination to establish a presence in the extensive industrial market.
In 2018, we acquired a shareholding in Halo Lasers UK and opened our own office in Malaysia to directly reach the Southeast Asian market.
The following two years, 2019 and 2020, are characterized by the launches of the SPA range and the improved version SPA2 (Smart Packaging Application): a wide range of CO2, fiber, and DPSS lasers covering marking and coding for all materials in the packaging market.
In 2022, BiDiLase was born, the ultra-fast laser capable of marking 100 unique QR codes per second and 1,000 meters per minute. A speed never before achieved by a coding laser in the market.
2023 is marked by the expansion of our own offices. Forbes Macsa is born, a joint venture in India with Forbes, one of the most powerful companies in the country, with whom we opened a manufacturing, planning, and sales plant to cover the Indian market. In the same year, we consolidate Macsa UK by acquiring 100% of Halo Lasers in England.

Innovation as a key point
Macsa ID is one of the 4 leading companies in the world in coding and marking lasers.
It offers the widest range of lasers to code and mark both in the productive sectors (food, beverages, pharmaceutical, healthcare, cosmetics …) as well as in the industrial ones (industry, automotive, aeronautics, defense, construction materials …).
Macsa ID is recognized as a world leader in technological innovation in lasers for marking and coding. The company invests more than 10% of its turnover in R&D every year.
It was the first company in the world to develop a dynamic coding laser. It has registered more than 30 international patents that have driven the most important technological developments in this industry, including the first dynamic coding laser for production lines (1990) and the first fibre laser for marking goods on production lines as well (2009).
Within its wide and innovative range, Macsa also has Lasertex, one of the few companies in the world which provides lasers for the textile industry. This type of technology helps to make the textile industry more sustainable and efficient.
2008: OSCAR EMBALLAGE for the Technological Innovation.
2008: BIEMH National Design Award.
2009: DAVOS Award for the most Innovative Project.
2011: Finalist INDUSTRIAL EXCELLENCE AWARD SPAIN IESE.
2012: 10th Edition Innovative Award Machine-Tool Finalist.
2013: PIMEC Award for the most competitive in the medium-size company category.
2013: EUROPE’S 500 AWARDS 2013-2014. Innovation category.
2014: EUROPE’S 500 AWARDS 2014-15 as the Best Company of the Year.
2015: CECOT : Recognition to the most international company. Nit de l’Empresari 2015.
2016/ 2017: European Bussiness Awards: Ruban d’Honneur, one of the 10 most innovative companies in Europe.
2018: CECOT : Recognition to the most international company. Nit de l’Empresari 2018.
2019: AEDEEC European Award as one of the most representative companies in the country.
2019: EUROPEAN BUSINESS AWARDS: The Award for Innovation Winner
2019
EUROPEAN BUSINESS AWARDS: The Award for Innovation Winner
2019
AEDEEC European Award as one of the most representative companies in the country.
2018
CECOT : Recognition to the most international company. Nit de l’Empresari 2018.
2016
2016/ 2017: European Bussiness Awards: Ruban d’Honneur, one of the 10 most innovative companies in Europe.
2015
CECOT: Recognition to the most international company. Nit de l’Empresari 2015.
2014
EUROPE’S 500 AWARDS 2014-15 as the Best Company of the Year.
2013
EUROPE’S 500 AWARDS 2013-2014. Innovation category.
2013
PIMEC Award for the most competitive in the medium-size company category.
2012
10th Edition Innovative Award Machine-Tool Finalist.
2011
Finalist INDUSTRIAL EXCELLENCE AWARD SPAIN IESE.
2009
DAVOS Award for the most Innovative Project.
2008
OSCAR EMBALLAGE for the Technological Innovation.
2008
BIEMH National Design Award.
Respect for the environment
Macsa ID lasers for coding and marking have a high technological component. We have invested in them a great deal of time and effort so that they are as effective for our customers as they are respectful of the environment. Our goal for the coming years is to make sustainability one of the key parts of our business strategy. By incorporating this concept into our business model, we can add value to everything we do.
Today’s consumers expect and demand new environmental and social values from companies and brands. The Havas World Report, March 2017, indicates that 75% of consumers believe that companies have an ethical obligation to operate in a sustainable manner without damaging the environment and 53% of them avoid buying from companies that have a negative social environmental impact. Lasers for coding and marking are more sustainable than any other technology:

In recent times, Macsa ID has joined the industry’s reflection on the need to create solutions that optimize production and profitability, while reducing their impact on the planet.
As a result of this reflection, Ecoding was born: a concept that brings together the most ecological and sustainable solutions from Macsa ID. In this lines, laser technology meets all the conditions to become the perfect solution in terms of sustainability:
• No harmful emissions that can affect people and the planet.
• No consumables: laser is a clean Technology that does not produce waste.
• Clean: it performs marking and coding without the intervention of consumables such as ink that make a mess of the workspace.
• Energy efficient: maximum speed and quality of coding and marking with optimal use of energy.
Improve the marking and coding of your company while reducing your environmental impact
Consumable free
Clean, waste free technology
Ecological
No harmful emissions. Good for the work environment and for the planet.
Clean
It will allow you to enjoy a cleaner and safer work space.
Energy efficient
Maximum quality and market speed with minimum energy consumption.